Hematopoietic cell transplant (HCT) is the only established cure for sickle cell disease (SCD). Engraftment (chimerism) of donor red blood cells (RBCs) determines HCT success but current methods measure only white blood cell chimerism and may be inaccurate or fail to indicate graft rejection in a timely manner. Measurement of differentially methylated regions (DMRs) in nucleated red blood cells (nRBCs) to distinguish individuals with sickle cell anemia (genotype HbSS) nRBCs from HbAA and HbAS nRBCs to quantify RBC chimerism could improve post-HCT monitoring by increasing sensitivity to detect rejection and allow for early intervention.
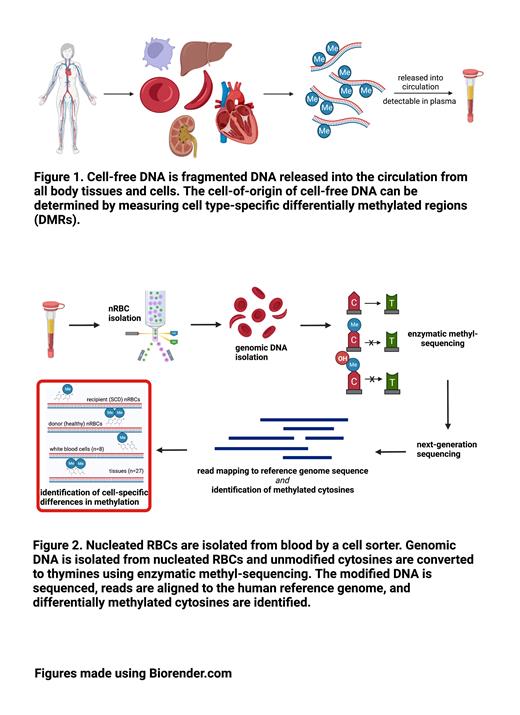
Cell-free DNA derived from nRBCs represents a potential method to specifically measure RBC chimerism. Methylation of cytosine nucleotides that are followed by a guanine (methylated CpG sites) is a major means of cell-specific gene expression, and measuring regions of multiple differentially methylated cytosines (DMRs) enables the identification of cell-of-origin of DNA fragments. Cell-free DNA is released into the circulation from all body tissues and cells, including nRBCs, and retains their cell-specific methylation signatures (figure 1). We hypothesized that DMRs unique to healthy and/or SCD nRBC DNA could be identified and later quantified to assess RBC donor chimerism.
We generated genome-wide methylomes of nRBC DNA from pediatric SCD (n=2) and non-SCD control (n=3) patients using enzymatic methyl-sequencing (figure 2). From these 5 novel nRBC methylomes and publicly available methylomes and hydroxymethylomes from 35 different tissues and hematopoietic cells we computationally identified 105 DMRs uniquely found in nRBCs compared to other tissues and cells. There were 34 DMRs that specifically distinguished control nRBCs from SCD nRBCs.
The 105 DMRs unique to nRBCs were all relatively hypomethylated. These DMRs were intergenic and not associated with CpG islands or shores. DMRs that distinguished SCD nRBCs from control nRBCs were predominantly hypermethylated (76%) in SCD nRBCs and were commonly (21%-24%) located at promoter, exon or intron sequences while 47% were intergenic. 24% were in CpG islands and 5% were in CpG shores.
The most significant gene ontology terms in SCD-specific nRBC DMRs were cardiac-related, including heart contraction and conduction. Other highly significant terms were related to cell signaling, transmembrane transporters, and ion channels. Enriched pathway analysis identified terms related to cell signaling, though these were associated with neuronal and immune signaling pathways.
To validate our in silico findings, we amplified 8 DMRs from genomic DNA isolated from 23 different cells and tissues and compared methylation patterns. Four DMRs were relatively hypomethylated in control nRBCs compared to SCD nRBCs and other tissues and cells. Amplification of these DMRs from cell-free DNA in patient plasma before and after HCT will validate them as potential unique markers of successful RBC chimerism. Quantification in recipients with HbAA and HbAS donors will be performed.
The successful identification of nRBC-specific DMRs in plasma cell-free DNA will enable novel methodology for the quantification of RBC chimerism after HCT for SCD that can be compared to conventional methods of chimerism measurement in future clinical trials.
Disclosures
Guilcher:bluebird bio, Inc.: Research Funding; Bristol Myers Squibb: Research Funding.